Abstract
Introduction: Gene therapy for people with hemophilia B appears to have a durable response, with data presented over 5 years (Samelson-Jones. Blood 2021; 138_suppl 1: 3975) and 8 years (Nathwani. Blood 2018; 132_suppl 1: 491) post-administration. Here we review the durability of AMT-060 and etranacogene dezaparvovec (formerly AMT-061) investigational gene therapies for hemophilia B, defined by sustained factor IX (FIX) activity levels and hemostatic protection. AMT-060 was developed as a precursor to etranacogene dezaparvovec. Both gene therapies are almost identical, comprising an adeno-associated virus serotype 5 (AAV5) vector containing a FIX transgene under the control of a liver-specific promoter; the transgene encodes wild-type FIX in AMT-060 and the highly active Padua variant of FIX in etranacogene dezaparvovec, differing by only one amino acid. Therefore, durability of response is expected to be similar for AMT-060 and etranacogene dezaparvovec.
Aim: Outline the observed durability of AMT-060 and etranacogene dezaparvovec in people with severe or moderately severe hemophilia B.
Methods: Three clinical trials are ongoing in adults with severe or moderately severe hemophilia B. Data have been captured over 5 years in the Phase 1/2 study assessing AMT-060 (N=10; Cohort 1: n=5, 5x1012 gc/kg ; Cohort 2: n=5, 2x1013 gc/kg; NCT02396342), 3 years in the Phase 2b study assessing etranacogene dezaparvovec (N=3, 2x1013 gc/kg; NCT03489291) and 2 years in the Phase 3 HOPE-B study assessing etranacogene dezaparvovec (N=54, 2x1013 gc/kg; NCT03569891). Study designs have been reported previously. All three studies assessed FIX activity levels and bleeding events.
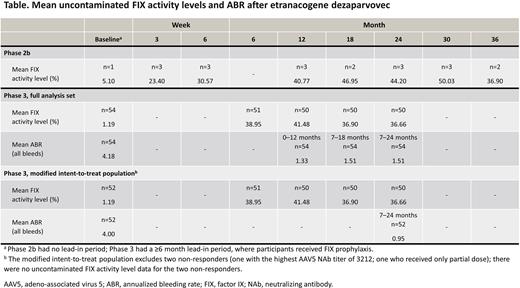
Results: In the Phase 1/2 AMT-060 study, FIX activity levels remained stable in both cohorts 5 years after dosing. Mean FIX activity was 4.4% for Cohort 1 at 52 weeks and 6.9% for Cohort 2 at 26 weeks; mean FIX activity after 5 years was 5.2% and 7.4%, for Cohorts 1 and 2, respectively. Greater FIX activity levels were achieved with etranacogene dezaparvovec. After 3 and 2 years of follow-up in the Phase 2b and 3 studies, respectively, mean FIX activity levels remained in the near-normal range (Table). In the Phase 2b study, mean FIX activity was 23.4% at Week 3 (n=3); this increased to 36.9% at Year 3 (n=2). A sustained mean FIX activity level was seen in the Phase 3 study: 38.95% at Month 6 (n=51) and 36.66% at Year 2 (n=50). This was observed in both the full analysis set (n=54) and in the modified intent-to-treat population (n=52) that excluded two non-responders (one with the highest AAV5 neutralizing antibody titer of 3212; one that received only a partial dose; Table).
With regards to hemostatic protection, in Year 1 post-AMT-060 administration, the mean annualized bleeding rate (ABR) was 7.30 in Cohort 1, and this decreased to 6.40 at Year 5. In Cohort 2, mean ABR decreased from 1.58 in Year 1 to 0.20 in Year 5. The etranacogene dezaparvovec studies showed a similar low bleeding rate. In the Phase 2b study, no bleeding episodes occurred between 2.5-3 years of follow-up. Overall, the ABR over 3 years was 0.22. Two bleeding episodes (one very mild, spontaneous bleed; one traumatic) occurred in one participant within 18 months of dosing; the participant received FIX product replacement therapy (1700 IU) after each bleed. In the Phase 3 study, ABR (all bleeds) achieved at Months 7-18 post-administration was 1.51 (reduced from 4.18 at baseline); this was maintained through to 2 years in the full analysis set (n=54; Table). A similar trend was seen when assessing the modified intent-to-treat population (n=52), with a reduction in ABR from 4.00 at baseline to 0.95 after 2 years of follow-up. Reductions in ABR for spontaneous, joint, and traumatic bleeds were also maintained through 2 years of follow-up.
No new safety signals have been identified after 5 years of follow-up for AMT-060 and up to 3 years of follow-up for etranacogene dezaparvovec.
Conclusions: Gene therapy for hemophilia B appears to have a durable response. Across the Phase 1/2 study of AMT-060 and the Phase 2b and Phase 3 studies of etranacogene dezaparvovec, FIX activity levels are sustained throughout the observation period. Reductions in bleeding events also remained stable across the course of the three studies. Similar durable responses have been observed in published literature for hemophilia B gene therapies.
Disclosures
Miesbach:Alnylam: Consultancy; uniQure: Consultancy; Sobi: Consultancy; Biomarin: Consultancy; Freeline: Consultancy; Takeda: Consultancy; Roche: Consultancy; LFB: Consultancy; Pfizer: Consultancy; Bayer: Consultancy; Biogen: Consultancy; Chugai: Consultancy; CSL Behring: Consultancy; Novo Nordisk: Consultancy; Octapharma: Consultancy. Recht:Foundation for Women and Girls with Blood Disorders; Partners in Bleeding Disorders: Thrombosis and Hemostasis Societies of North America: Membership on an entity's Board of Directors or advisory committees; Bayer, Biomarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, uniQure: Research Funding; Catalyst Biosciences, CSL Behring, Genentech, Grifols, Hema Biologics, Novo Nordisk, Pfizer, Sanofi, Takeda, uniQure: Consultancy; American Thrombosis and Hemostasis Network; Yale University School of Medicine: Current Employment; Oregon Health & Science University: Ended employment in the past 24 months. Key:Biomarin: Membership on an entity's Board of Directors or advisory committees; uniQure / CSL Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees. Sivamurthy:CSL Behring: Current Employment. Monahan:CSL Behring: Current Employment. Pipe:Takeda: Consultancy; Bayer: Consultancy; BioMarin Pharmaceutical Inc.: Consultancy; CSL Behring: Consultancy; HEMA Biologics: Consultancy; Regeneron/Intellia: Consultancy; ASC Therapeutics: Consultancy; Freeline: Consultancy; Apcintex: Consultancy; UniQure: Consultancy; Spark Therapeutics: Consultancy; Sanofi: Consultancy; Sangamo Therapeutics: Consultancy; Roche/Genentech: Consultancy; Pfizer: Consultancy; Novo Nordisk: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal